Home • Policy and Outreach • Availability of Veterinary Medicines
Access to and the availability of veterinary medicines is critical to keeping our pets and food-producing animals healthy.
Canada currently faces a number of challenges with respect to maintaining and improving access to the veterinary medicines needed to keep our animals healthy..

A Drug Identification Number (DIN) is a computer generated eight digit number assigned to a drug product by Health Canada prior to that product being marketed in Canada.
All licensed drug products in Canada have a unique DIN.
Marketed = a product that is currently being sold in Canada
Dormant = a product that was
previously marketed in Canada but for which there have been no sales for at least 12 months
Cancelled = a product that has left the Canadian market and is no longer available. DINS can be cancelled both before (pre-market) and after (post-market) a product is actually sold in Canada
A recent decline in the number of veterinary drugs available on the Canadian market is negatively impacting animal welfare, the quality of veterinary medicine practiced in Canada, and the sustainability and competitiveness of the Canadian livestock industries.
Current strategies used by veterinarians and producers to access products not available in Canada, such as use of compounded products, off-label drug use, own-use importation and online purchases from other countries, can result in serious risks to animal health, environmental safety and food safety in Canada, potentially leading to major impacts on trade and the closure of international borders to Canadian livestock exports.
Animal health products like veterinary medicines are critical tools for the sustainable production of safe food. The health of animals is inextricably linked to human health and the well-being of the planet.
The ability of the animal health industry to innovate and develop new products within the Canadian regulatory environment will in large part dictate Canada’s success in achieving the desired outcomes of the Pan-Canadian Action Plan on Antimicrobial Resistance and our collective efforts to achieve the UN Sustainable Development Goals by 2030.
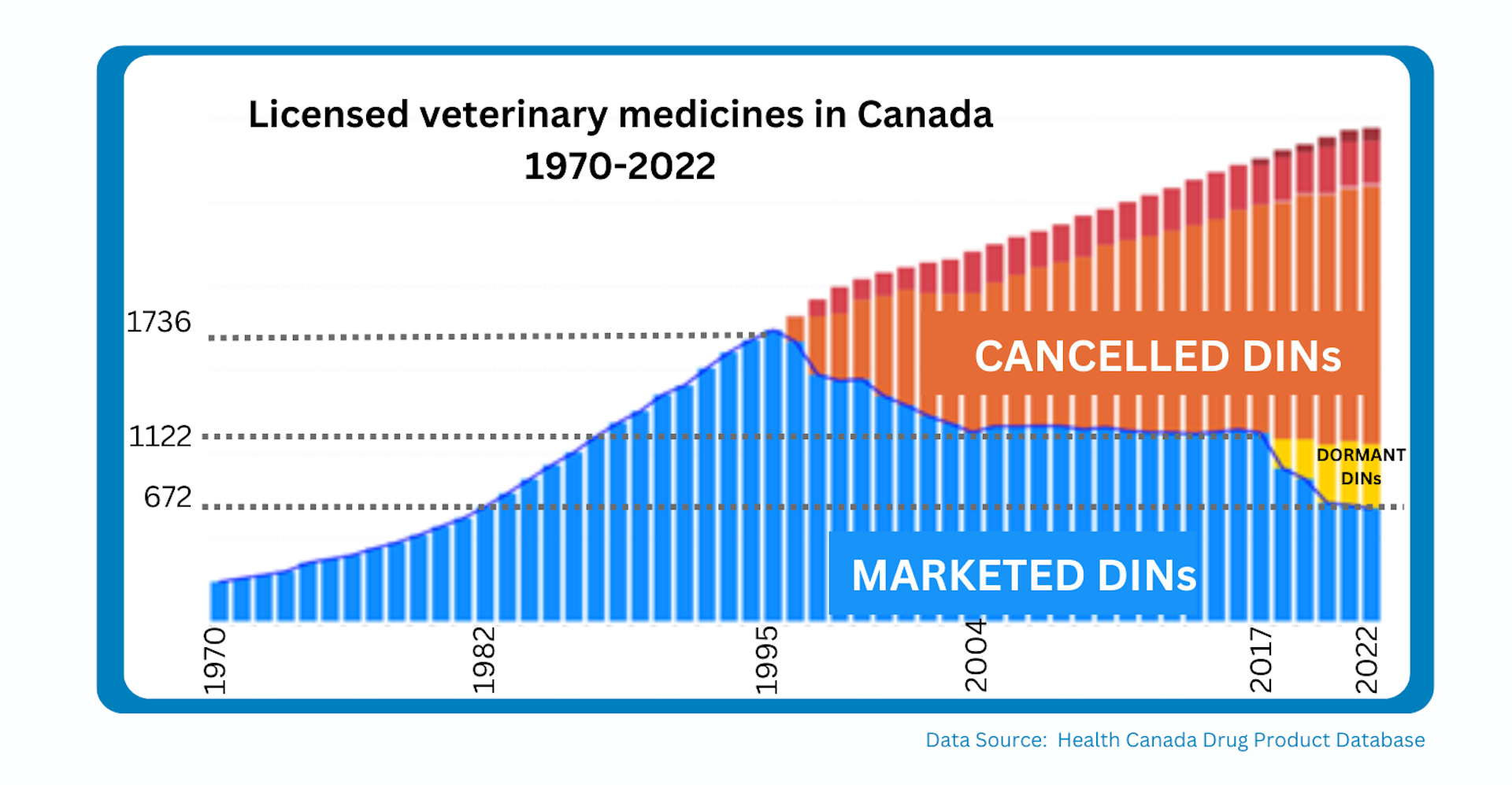
As of the end of 2023, a total of 399 DINs were sitting in dormant status and are at risk of permanently leaving the Canadian market. They serve as red flags for products which are at high risk of moving to a cancelled DIN status and becoming permanently unavailable in Canada. The Canadian marketplace already faces significant challenges due to the nature of our highly regulated yet very small market, and Canada is falling behind our global competitors with respect to product availability.
CAHI remains committed to working with our regulators and politicians to address this growing concern. CAHI strongly urges the Government to develop and implement a strategy to increase the availability of veterinary medicines in the Canadian market.